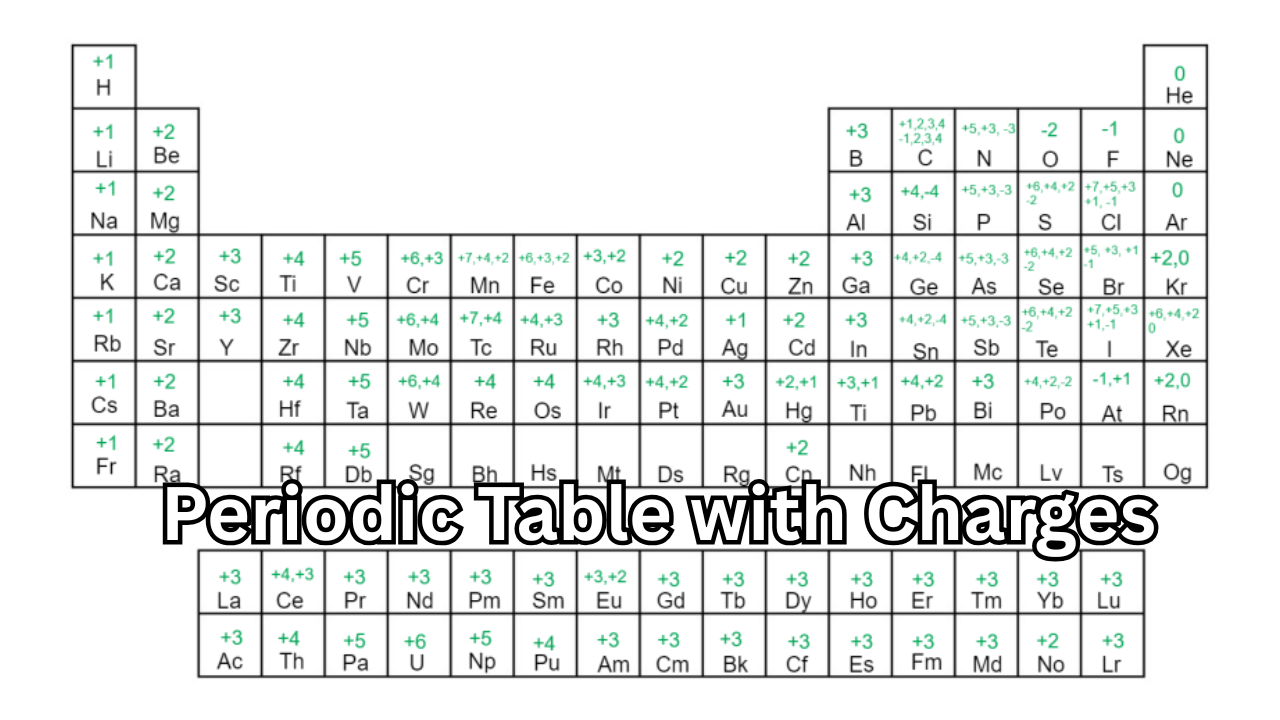
Introduction to the Periodic Table with Charges
The periodic table with charges is one of the most powerful tools in chemistry. It allows students, teachers, and scientists to easily identify how elements form ions by gaining or losing electrons. Understanding these charges—also known as oxidation states—helps explain why certain elements bond together and how chemical reactions occur.
In this detailed guide, we’ll explore the meaning of charges in the periodic table, how to read and use them, why they’re important for predicting chemical behavior, and how mastering this concept can make learning chemistry simpler and more effective.
What Are Charges in the Periodic Table?
In chemistry, a charge (or oxidation number) represents the electrical nature of an atom after it loses or gains electrons. Since atoms always strive to become stable by having a full outer shell of electrons, they either:
- Lose electrons to form positive ions (cations)
- Gain electrons to form negative ions (anions)
For example:
- Sodium (Na) loses one electron to form Na⁺.
- Chlorine (Cl) gains one electron to form Cl⁻.
These charges are what determine how elements react and bond with others, forming stable compounds like NaCl (table salt).
How the Periodic Table with Charges Works
The periodic table is arranged in groups (columns) and periods (rows). Elements in the same group have the same number of valence electrons and usually form ions with the same charge.
Here’s how charges are distributed across the periodic table:
- Group 1 (Alkali Metals): +1 charge (e.g., Li⁺, Na⁺, K⁺)
- Group 2 (Alkaline Earth Metals): +2 charge (e.g., Mg²⁺, Ca²⁺)
- Group 13: +3 charge (e.g., Al³⁺)
- Group 15: −3 charge (e.g., N³⁻, P³⁻)
- Group 16: −2 charge (e.g., O²⁻, S²⁻)
- Group 17 (Halogens): −1 charge (e.g., F⁻, Cl⁻, Br⁻)
- Group 18 (Noble Gases): 0 charge (inert elements that don’t form ions)
Transition metals, located in the middle of the periodic table, can have multiple possible charges—a feature that makes their chemistry more complex and fascinating.
Importance of Charges in Chemistry
Understanding the charges of elements is essential because it explains how and why chemical reactions happen. Here’s why it matters:
- Predicting Compound Formation – By knowing the charges, you can predict how elements combine. For example, calcium (Ca²⁺) and chlorine (Cl⁻) form CaCl₂.
- Balancing Chemical Equations – Charges help ensure the total positive and negative ions are balanced in a reaction.
- Understanding Acids and Bases – The behavior of hydrogen ions (H⁺) and hydroxide ions (OH⁻) determines acid-base reactions.
- Redox Reactions – Knowing changes in oxidation states helps identify which elements are oxidized and reduced.

How to Remember Common Element Charges
Learning the periodic table with charges might seem tough at first, but with the right methods, it becomes easy. Here’s how you can memorize them quickly:
- Use group numbers – Elements in the same group have similar charges.
- Practice writing ionic compounds – Examples like NaCl, CaCl₂, and Al₂O₃ reinforce charge balance.
- Create flashcards – Write element symbols on one side and their charges on the other.
- Study in pairs – Learning which ions attract helps strengthen memory through association.
Transition Metals and Variable Charges
Transition metals, unlike main-group elements, often form ions with more than one possible charge. This happens because they can lose different numbers of electrons from their outer orbitals.
Examples include:
- Iron (Fe): Fe²⁺ and Fe³⁺
- Copper (Cu): Cu⁺ and Cu²⁺
- Chromium (Cr): Cr²⁺ and Cr³⁺
When naming compounds that contain transition metals, we use Roman numerals to indicate their charge:
- FeCl₂ → Iron(II) chloride
- FeCl₃ → Iron(III) chloride
This naming system makes it clear which ion is being referred to.
The Role of Charges in Chemical Bonding
The charge of an element determines how it bonds with others. There are two main types of chemical bonds influenced by charges:
- Ionic Bonds – Formed between metals and nonmetals. Metals lose electrons (forming positive ions), while nonmetals gain electrons (forming negative ions). The attraction between them creates an ionic compound.
- Example: Na⁺ + Cl⁻ → NaCl
- Covalent Bonds – Formed between nonmetals that share electrons to achieve stability. While these don’t create full charges, the distribution of electrons is still influenced by each atom’s electronegativity.
- Example: H₂O (Water)

Applications of the Periodic Table with Charges
The periodic table with charges isn’t just a classroom concept—it has numerous real-world applications, including:
- Industrial Chemistry: Helps predict reaction products and design compounds.
- Pharmaceuticals: Understanding ion interactions aids in drug formulation.
- Environmental Studies: Used in analyzing pollution and water chemistry.
- Materials Science: Helps design alloys and advanced materials with specific properties.
Why Every Student Should Learn the Periodic Table with Charges
For chemistry students, the periodic table with charges is a must-know reference. It simplifies chemical equation writing, helps identify compound formulas, and improves understanding of atomic behavior.
Having a periodic table that includes charges helps in:
- Quick recall during exams.
- Faster problem-solving in labs.
- Understanding patterns and trends in element reactivity.
Final Thoughts on the Periodic Table with Charges
The periodic table with charges is much more than a scientific chart—it’s a roadmap to understanding how elements behave in the universe. By mastering the concept of ionic charges, students and scientists can predict chemical bonding, reaction outcomes, and molecular stability.
Learning these charges builds a strong foundation in chemistry, making complex topics like redox reactions, acid-base chemistry, and molecular bonding easier to grasp.
Whether you’re a beginner or an advanced chemistry enthusiast, the periodic table with charges will always be your most valuable guide in exploring the fascinating world of elements and their reactions.
Also Read: McCrispy: The Irresistible McDonald’s Chicken Burger Everyone Is Talking About





1 thought on “Periodic Table with Charges: Understanding Element Oxidation States, Ionic Behavior, and Chemical Bonding for Easy Learning”
Comments are closed.